


Decimal value for the percentage of C-13 is 0.02 (obtained by dividing 2 from 100).Decimal value for the percentage of C-12 is 0.98 (obtained by dividing 98 from 100).Let us calculate the atomic weight of carbon using the atomic masses of these isotopes. At last, add the answers together to get the final answer.Įxample: Suppose we have 98% of C-12 isotope and 2% of the C-13 isotope in nature.Next, multiply the atomic masses of each isotope from these decimal values accordingly.First, convert the percentages into decimal values by dividing them with 100.We can use the following two steps for this calculation The atomic weight that we see in the periodic table are calculated according to this phenomenon.
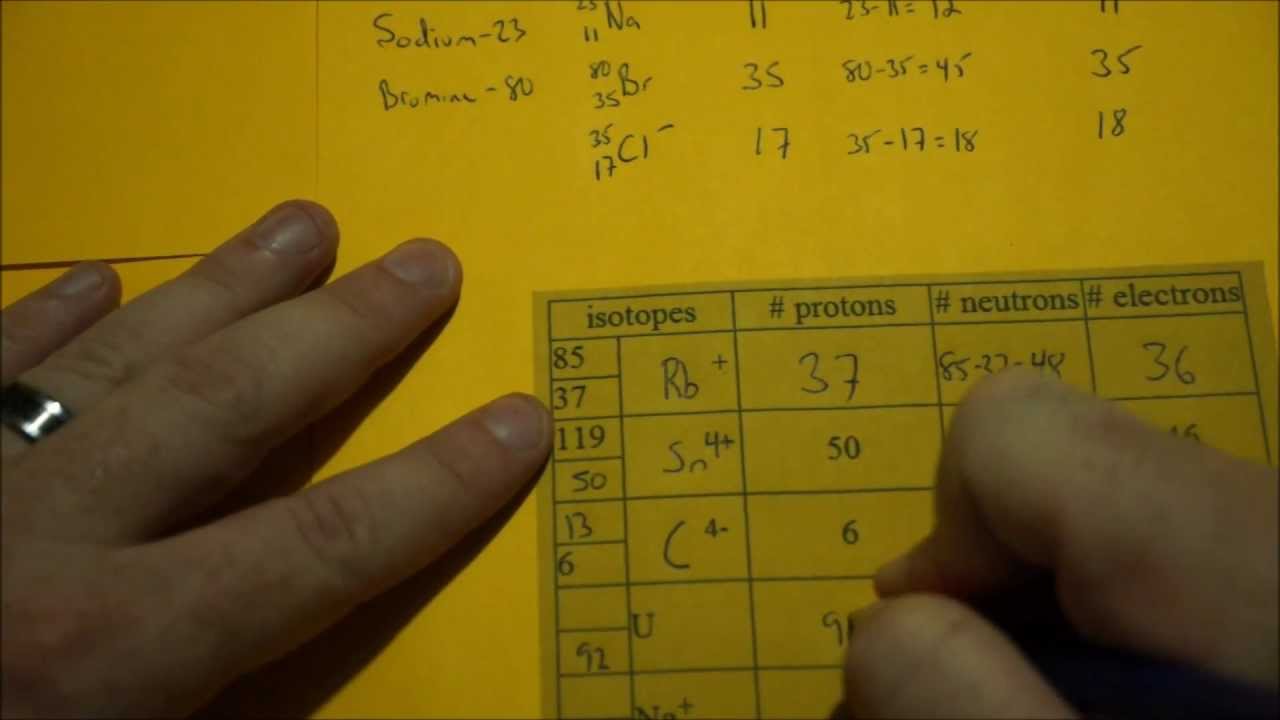
There, we can calculate the average mass using the atomic masses of each isotope to get the atomic weight. We need to consider the atomic masses of all the isotopes and their percentages when getting the atomic weight of a chemical element. There are different percentages of different isotopes that occur in nature. Isotopes have the same number of protons (which makes them belong to the same chemical element) and different numbers of neutrons in the atomic nucleus. Most of the times, chemical elements have isotopes isotopes are the different forms of the same chemical element. Side by Side Comparison – Atomic Weight vs Atomic Mass in Tabular FormĪtomic weight is the average weight of an element, with respect to all its isotopes and their relative abundances. However, they bear different meanings, and it causes a significant error in bulk material calculations if we take these two terms as one. Most people use the terms atomic mass and atomic weight interchangeably. The key difference between atomic weight and atomic mass is that atomic weight is the average weight of an element, with respect to all its isotopes and their relative abundances but, atomic mass is the mass of a single atom.


 0 kommentar(er)
0 kommentar(er)
